The first trial was started in the age group of 12 to 18 years followed by the age group of 6 to 12 while trials for children between the age of 2-6 years are currently underway. On the other hand Covid-19 clinical trials using therapeutics continue to decrease.
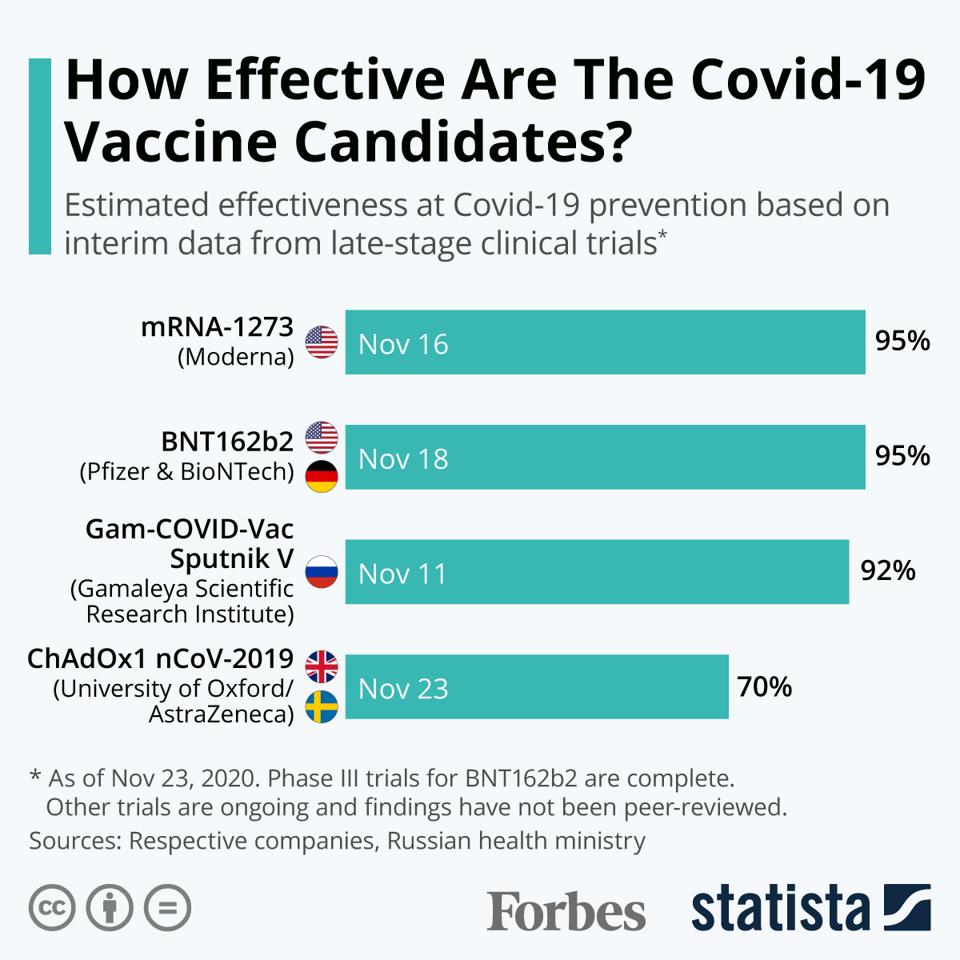
How Effective Are The Covid 19 Vaccine Candidates Infographic
COVID-19 Clinical Trials.

What covid 19 vaccines are in clinical trials. The absence of reported absolute risk reduction in COVID-19 vaccine clinical trials can lead to outcome reporting bias that affects the interpretation of vaccine efficacy. An order has been issued ICMR Indian Council of Medical Research has been asked to share a list of those who participated in the. CureVacs Covid-19 Vaccine Disappoints in Clinical Trial A preliminary analysis showed that CureVacs mRNA vaccine had an efficacy of just 47 percent.
Zydus Cadilas ZyCoV-D vaccine will be a three-dose vaccine shot and if approved it will be the worlds first plasmid DNA vaccine. List of COVID-19 treatment trials. Learn more about US.
Herein we provide operational COVID-19 vaccine guidance for patients participating in oncology clinical trials. VBI Vaccines has reported preliminary data from Phase I part of its Phase III clinical trial where its Covid-19 vaccine candidate VBI-2902a induced potent immune responses in healthy adult subjects. COVID-19 mRNA vaccines directly inject cells with a synthetic genetic code to replicate the spike S protein found on the surface of the coronavirus SARS-CoV-2.
This is pretty devastating for them one. The Janssen COVID-19 vaccine also referred to as the Johnson Johnson vaccine or Ad26COV2-S the Moderna COVID-19 vaccine also known as mRNA-1273 and the Pfizer-BioNTech. It will include approximately 150 individuals who already have received one of the three COVID-19 vaccine regimens currently available under FDA Emergency Use Authorization in the United States.
This may be due to the increase in the availability of vaccines and the negative data in clinical trials from some therapeutics such as hydroxychloroquine. As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States. Clinical trials offer hope for many people and provide an opportunity to help researchers find new or improve existing treatments.
A monovalent and enveloped virus-like particle eVLP Covid-19-specific vaccine VBI-2902 expresses the SARS-CoV-2 spike protein. Clinical trials use volunteers who agree to participate in these types of studies. 27 julio 2021.
COVID-19 vaccine clinical trials including vaccines in earlier stages of development by visiting clinicaltrialsgov. Clinical trials are medical research studies to test new ways to prevent detect or treat diseases. Protein based inactivated vaccines protein subunit VLP and T-cell based vaccines gene based DNA or RNA vaccines replicating or non-replicating viralbacterial vectored vaccines and a combination of both protein-based and gene-based live-attenuated virus vaccines.
Novavax COVID-19 vaccine. If the outcome of the risk assessment is that a COVID-19 vaccine given to a trial subject is considered as a simple concomitant medication with no interaction that requires advice on timing of the. The present article uses clinical epidemiologic tools to critically appraise reports of efficacy in PfzierBioNTech and Moderna COVID-19 mRNA vaccine clinical trials.
Allows users to search for COVID-19 vaccines through various criteria such as vaccine platform schedule of vaccination route of administration developer trial phase and clinical endpoints. The number of clinical trials peaked in. NVX-CoV2373 clinical trials have enrolled more than 30000 volunteers around the globe.
Preliminary report of an open-label and randomised phase 1 clinical trial. 89 rows On this page. List of COVID-19 vaccine trials.
Currently COVID-19 candidate vaccines can be classified into three camps. Includes information on key attributes of each vaccine candidate and. In our perspective continued quality oncological care requires that patients with cancer including those involved in trials be prioritized for COVID-19 vaccination which should not affect trial eligibility.
NVX-CoV2373 is a protein-based vaccine engineered from the genetic sequence of SARS-CoV-2 the virus that causes Covid-19. Completed clinical trials data for Covid-19 vaccines will be unblinded and people who have obtained the jab will be given a certificates via the federal governments CoWIN platform an official mentioned. The database is updated regularly - twice a week Tuesday and Friday 1700 CET.
Once replicated the spike protein is proposed to trigger an immune response that creates antibodies against the virus. Safety tolerability and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine Ad5-nCoV in adults.

How Have Vaccines For Covid 19 Been Developed So Fast British Society For Immunology
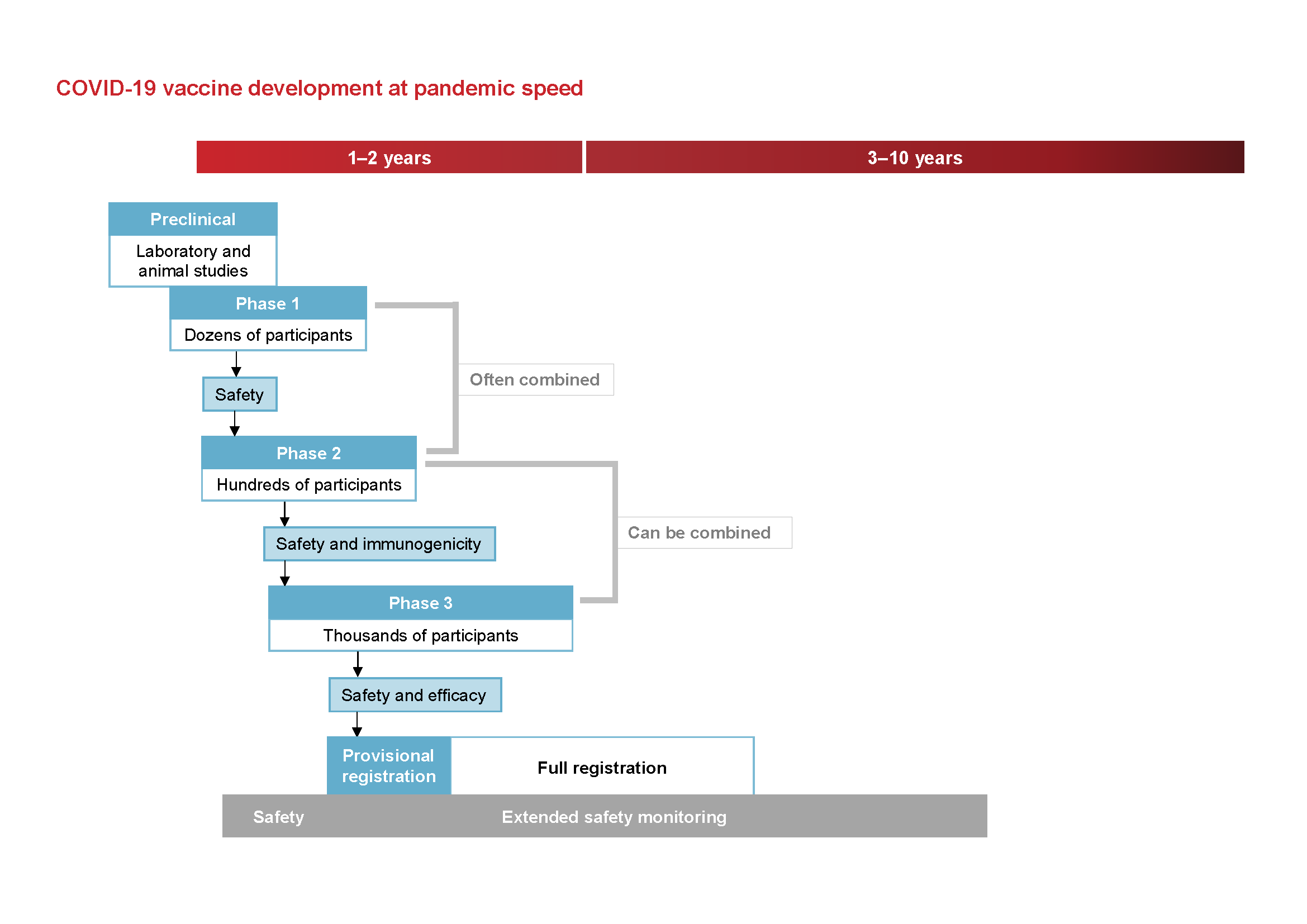
Phases Of Clinical Trials Ncirs

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 15 07 2021

Coronavirus Covid 19 Vaccines And Vaccination Campaign Statista
Get To Know The Covid 19 Vaccines Main Infographic Health Mil
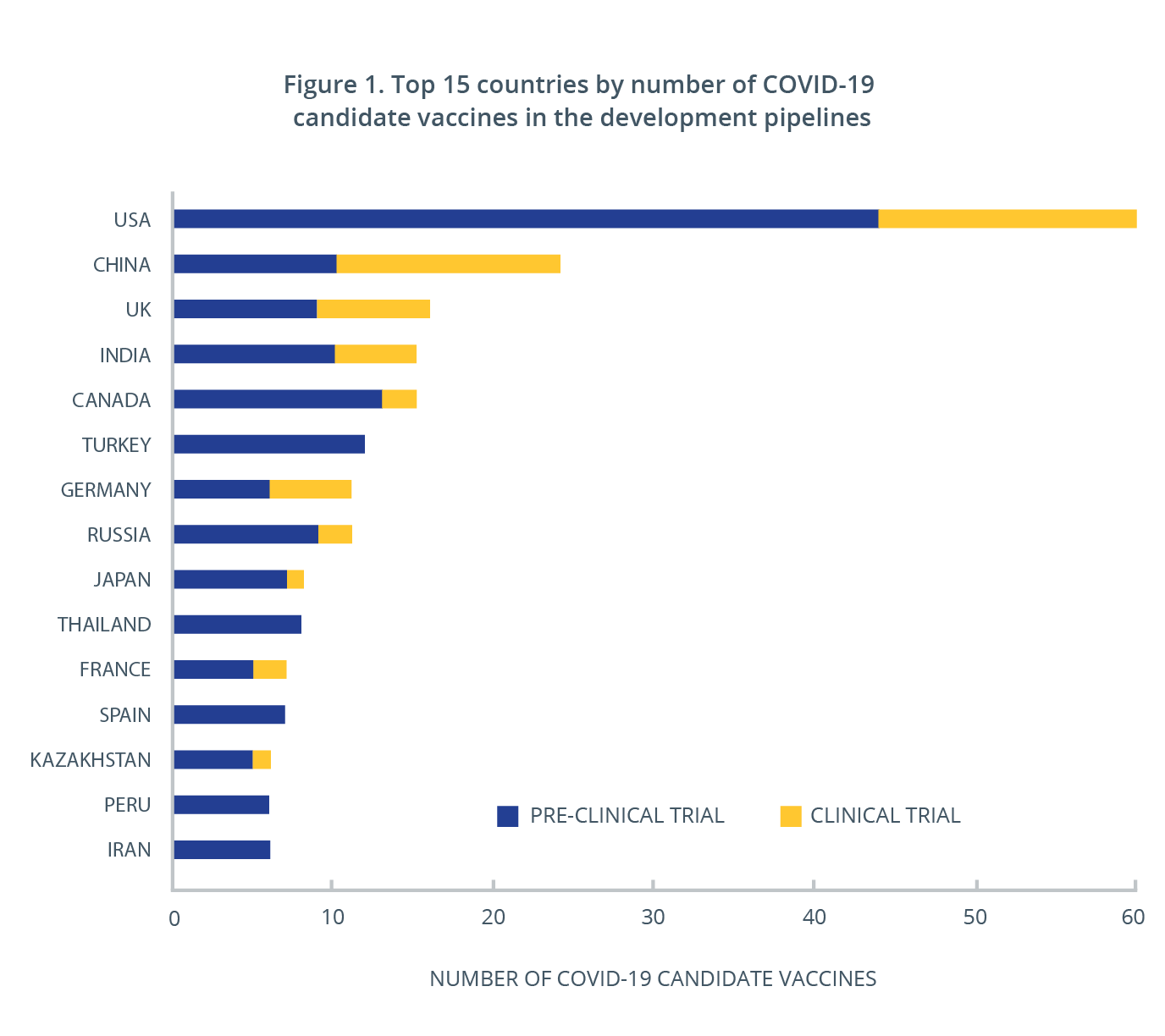
Top 5 Covid 19 Vaccine Questions Answered Cas

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 15 07 2021

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 15 07 2021
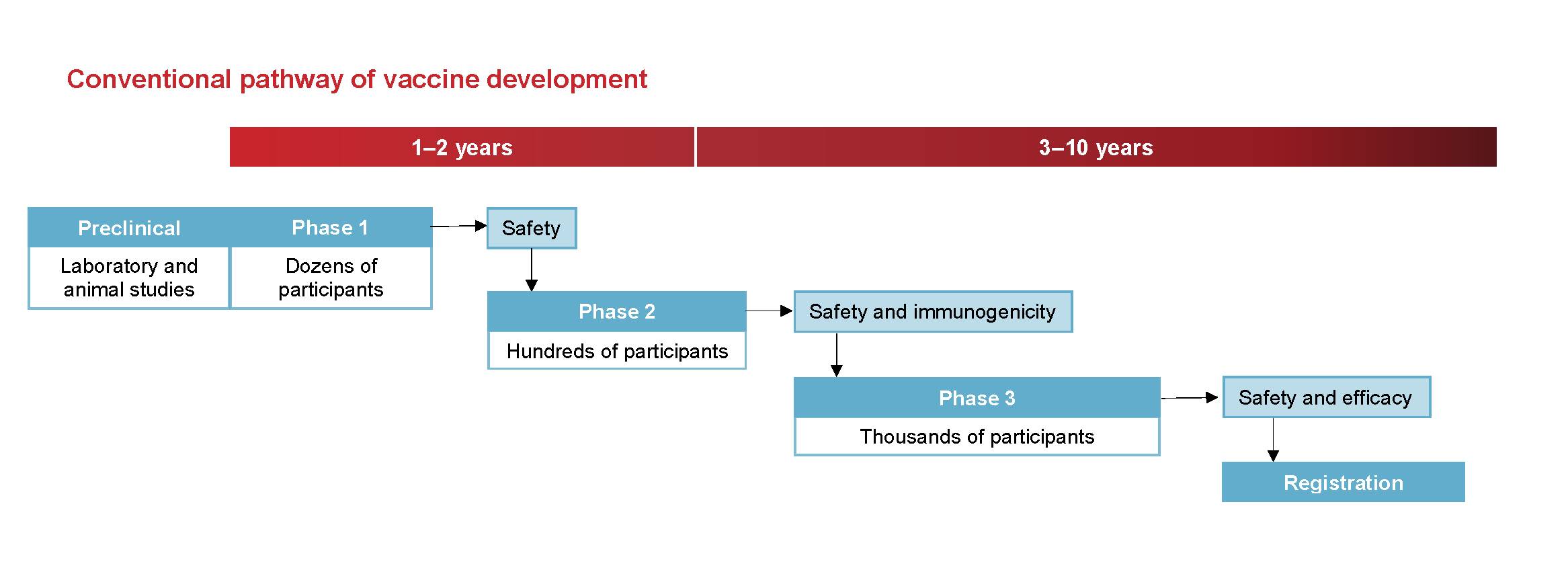
Phases Of Clinical Trials Ncirs

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 15 07 2021

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 15 07 2021
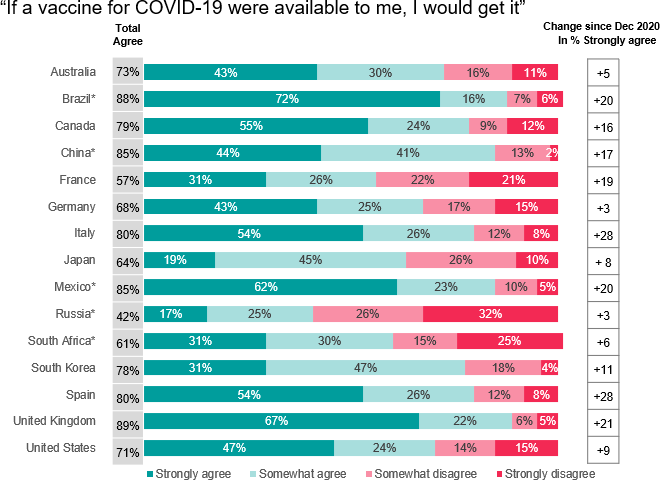
Global Attitudes Covid 19 Vaccines Ipsos
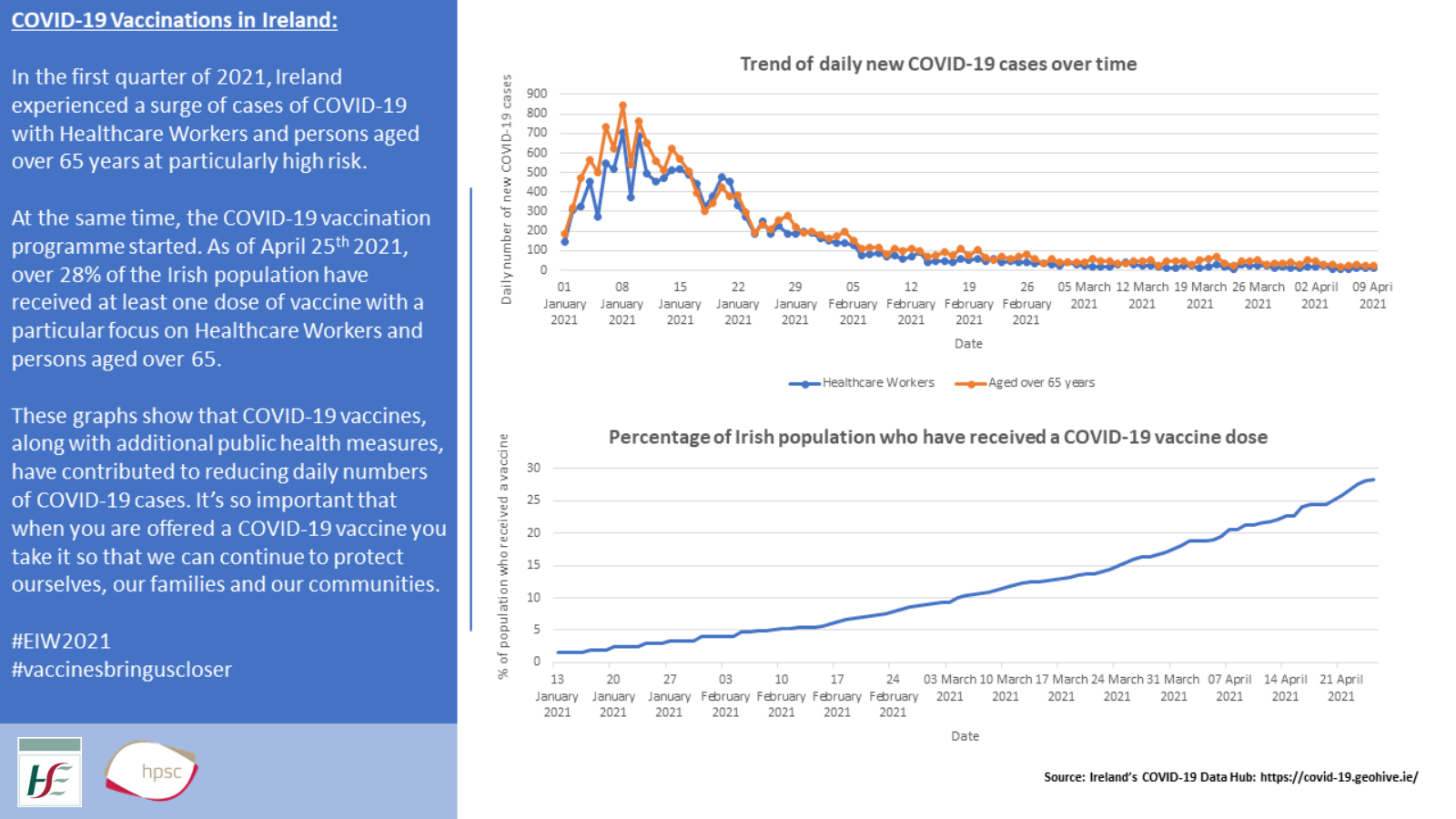
Covid 19 Vaccine Studies Hse Ie

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 15 07 2021

Worldimmunizationweek 2021 Everything You Need To Know About Covid 19 Vaccines Speaking Of Research
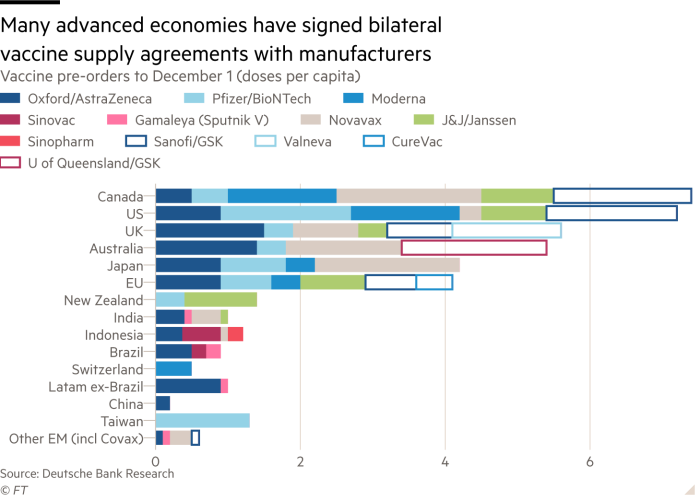
Indonesia S Covid 19 Vaccine Diplomacy The Challenge Of Public Access To Equitable Vaccines And Healthcare Services Indonesia For Global Justice
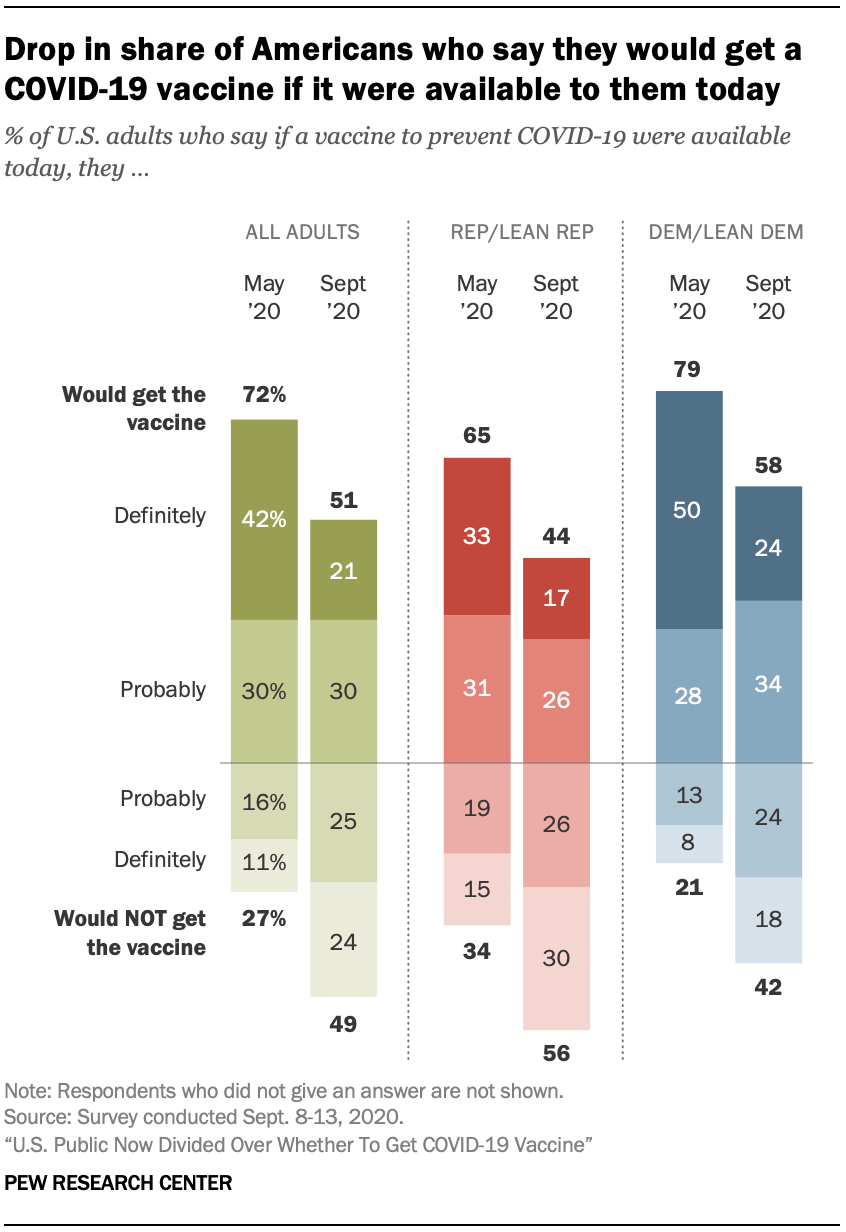
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
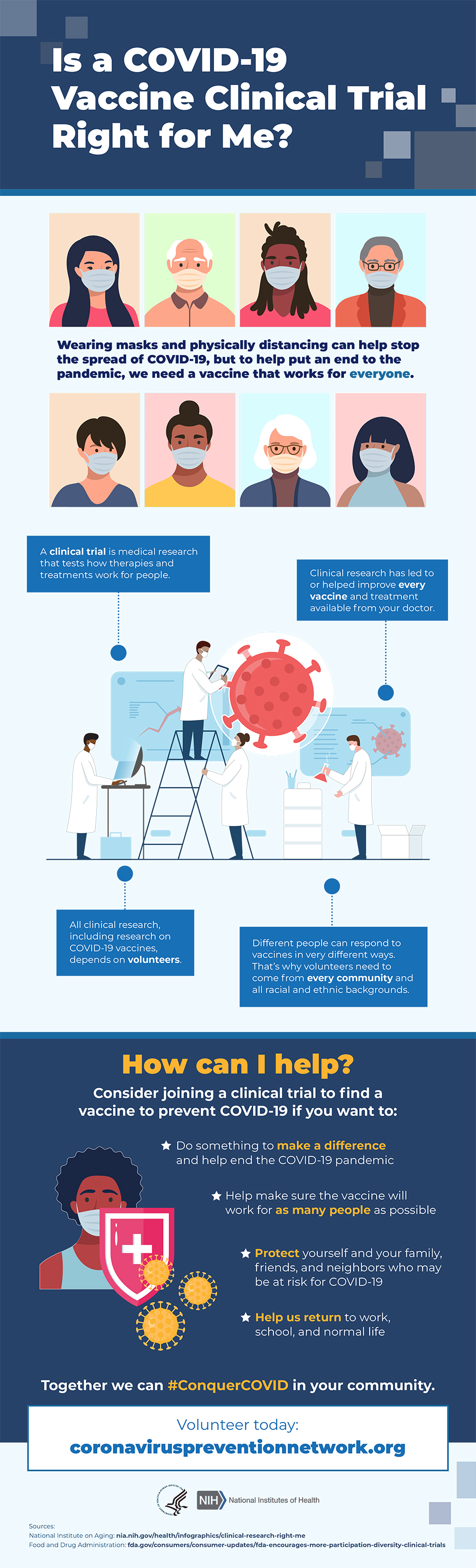
Clinical Trials Nih Covid 19 Research

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 15 07 2021

0 Comments